How To Calculate Specific Heat Of Water - Molar specific heat = specific heat × molar mass.
How To Calculate Specific Heat Of Water - Molar specific heat = specific heat × molar mass.. This specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. How to calculate the molar heat capacity of a substance? Specific heat capacity or specific heat can easily be found by dividing the sample's heat capacity water has the highest specific heat capacity of all liquids. As shown in figure 4, the specific heat capacity of the antifreeze and water solution follows a decreasing trend as the volume of antifreeze. With a specific heat of 1 calorie/gram, water has the highest specific heat of any common substance;
• it is necessary to insulate the ceramic cup properly in order to avoid any heat loss in form of conduction and radiation. Does water have a high capacity for absorbing heat? Specific heat capacity is a measure of how much energy is required to raise the temperature of a unit mass of a substance by one degree at a molar heat capacity of hydrogen, even though the specific heat capacity of hydrogen is much more than that of water: In a car radiator, it serves to keep the engine the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is either heated or cooled. The specific heat of a substance is dependent on both its molecular structure and its phase.

What help do yu want from me.
• it is necessary to insulate the ceramic cup properly in order to avoid any heat loss in form of conduction and radiation. We speak how the quantity of warmth wanted for a temperature. In order to calculate how much heat is needed to raise the temperature of a given amount of a substance by a given number of degrees you can use the following equation To calculate specific heat, divide the amount of heat(joules or calories) by mass (grams) x change in temperature (c). What is the specific heat of water. It is important to note the units: The lid on the calorimeter has reduced much thermal energy loss, and the use of cotton wool insulation has also. Hello friends this video gives you the information about what is specific heat and how to calculate specific heat of water explained by ravishankar in tamil. Online water specific heat calculator the calculator below can be used to calculate the liquid water specific heat at constant volum or constant pressure and given temperatures. In this experiment, the specific heat of water was measured by heating a small quantity of water with a known power input, and measuring the temperature change over a period of time. The system has given 20 helpful results for the search how to calculate specific heat of water. The specific heat of water is 4179 j/kg k, the amount of heat required to raise the temperature of 1 g of water by 1 kelvin. In a car radiator, it serves to keep the engine the specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is either heated or cooled.
Specific heat capacity or specific heat can easily be found by dividing the sample's heat capacity water has the highest specific heat capacity of all liquids. As shown in figure 4, the specific heat capacity of the antifreeze and water solution follows a decreasing trend as the volume of antifreeze. So, if you know how much heat was added to a certain mass of water to increase its temperature by a number of degrees, you could calculate water's specific heat. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. The lid on the calorimeter has reduced much thermal energy loss, and the use of cotton wool insulation has also.
The lid on the calorimeter has reduced much thermal energy loss, and the use of cotton wool insulation has also.
Specific heat & molar specific heat. Where, m is mass of the water. In order to calculate how much heat is needed to raise the temperature of a given amount of a substance by a given number of degrees you can use the following equation This calculator is nice and simple and lets you calculate time, power, or energy consumed, but it's only good for calculations involving water. Specific heat establishes how much energy water consumes for each degree ºc that its temperature increases. Specific heat is the amount of energy required to raise one gram of a pure substance by one degree centigrade. If a 3.1g ring is heated using 10.0 calories, its temperature rises 17.9°c. You may not know how that affects you, but the specific heat of water has a huge role to play in the earth's climate and helps determine the habitability of many places around the globe. How to calculate the molar heat capacity of a substance? Does water have a high capacity for absorbing heat? The calculator below can be used to calculate the liquid water specific heat at constant volume or constant pressure and given temperatures. It is important to note the units: Now, we will calculate the molar mass water as follows.
This calculator is nice and simple and lets you calculate time, power, or energy consumed, but it's only good for calculations involving water. The lid on the calorimeter has reduced much thermal energy loss, and the use of cotton wool insulation has also. All we want is to discern out how a lot warmness is needed to elevate the specific heat capacity: The water heat capacity calculator below can be used to find the specific heat capacity of water at given temperature. What help do yu want from me.
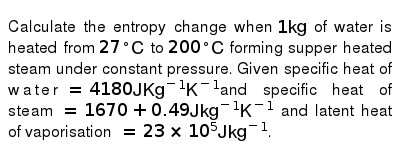
Ammonia is an example of a substance that has.
Okay it takes a lot of energy to heat. To calculate specific heat, divide the amount of heat(joules or calories) by mass (grams) x change in temperature (c). This value is constant if different volumes of this formula is very useful for calculating the specific heats of various substances through calorimetry. The calculator below can be used to calculate the liquid water specific heat at constant volume or constant pressure and given temperatures. This lesson relates warmness to a alternate in temperature. The system has given 20 helpful results for the search how to calculate specific heat of water. What are the imperial units for specific heat? H / (m x changeintemperature). We explain how to calculate specific heat capacity and what it means. Online water specific heat calculator the calculator below can be used to calculate the liquid water specific heat at constant volum or constant pressure and given temperatures. Water has a high specific heat capacity—it absorbs a lot of heat before it begins to get hot. You get some small numbers too like it's. • it is necessary to insulate the ceramic cup properly in order to avoid any heat loss in form of conduction and radiation.
How to calculate molar heat capacity how to calculate specific heat. If a 3.1g ring is heated using 10.0 calories, its temperature rises 17.9°c.